Metal-Ligand Complexes
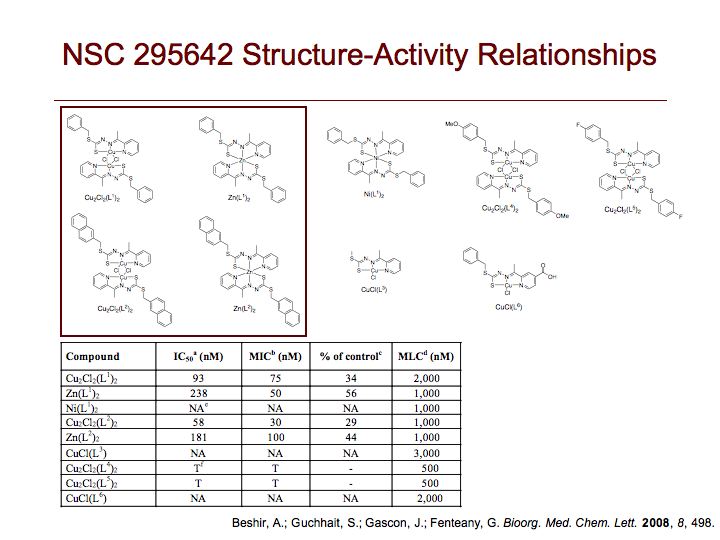
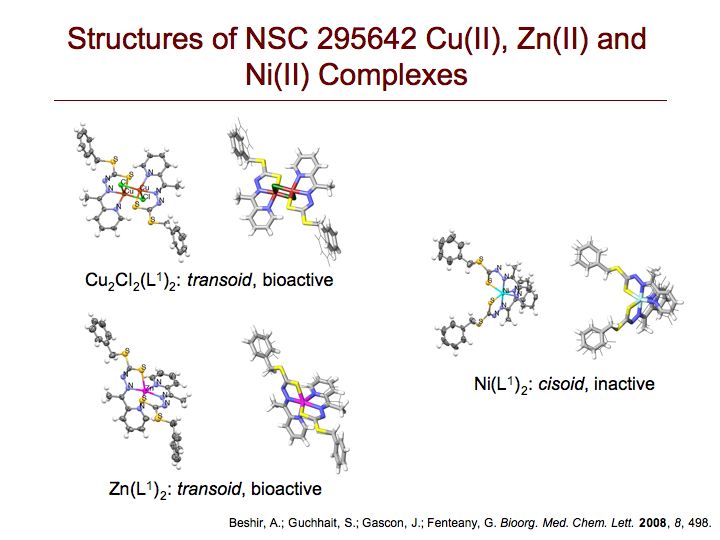
We screened the National Cancer Institute Diversity Set compound collection for small molecules that affect mammalian cell migration and identified NSC 295642 as an inhibitor of cell motility with nanomolar potency. We found by LC-MS and X-ray crystallography that NSC 295642, a Cu(II) complex of the Schiff base product of condensation of S-benzyl dithiocarbazate and 2-acetylpyridine, has a bridged dimeric Cu2Cl2(L)2 structure with distorted square pyramidal geometry. Each of the two copper atoms is five-coordinated to one of the two tridentate chelating ligands and both bridging chlorine atoms. To define structure-activity relationships, we investigated the bioactivity of related metal-ligand complexes derived from different metal(II) atoms and different ligands. Complexation of the NSC 295642 ligand with Zn(II) or Ni(II), delivered as metal(II) chloride salts under conditions identical to those used for preparation of the original Cu(II) complex, instead results in distorted octahedral bis-chelate structures, where a single metal atom is six-coordinated to two ligands. The Zn(L)2 complex possesses a potency similar to that of the Cu2Cl2(L)2 complex, while the Ni(L)2 has no antimigratory activity at all. We carried out density functional theory calculations to obtain the electronic ground state geometry of the complexes, both in vacuum and implicit water solvent. The X-ray crystal and energy-minimized structures are very similar and exhibit a transoid orientation of the S-benzyl groups relative to the central metal-coordinated rings for both of the bioactive Cu2Cl2(L)2 and Zn(L)2 complexes, despite their different coordination geometries. In contrast, the biologically inactive Ni(L)2 complex adopts a cisoid conformation. Varying the ligand structure, we found that hydrophobic S-alkylaryl groups are required for activity. Complexes with a simple S-methyl group, S-benzyl groups with polar substitutions or a carboxylated pyridine ring exhibit dramatically reduced activity. We tested the most potent metal-ligand complex in a number of cancer cell lines and found cell-type selectivity in its effect on cell motility. Collectively, these results suggest that a two-ligand structure with bulky nonpolar S-substituents in a transoid conformation is important for the antimigratory activity of these metal-ligand complexes.